- Clinical Research Trial (CRT)
- A clinical research trial (CRT) is a research in which an experimental design is used. In such a design the results of an experimental and a control group are compared to each other.
- Using a placebo
- Related topics to clinical research design:
- This manual helps you to do scientific research:
- Mission

Clinical Research Trial (CRT)
A clinical research trial (CRT) is a research in which an experimental design is used. In such a design the results of an experimental and a control group are compared to each other.
A Clinical Research Trial is highly valued by scientists. It compares the data from an experimental and a control group before and after an event occurred. This design is represented in a graphical model such as:
t1 t2
Group A R or M O1 X O2
Group B R or M O1 O2
It simply says: this research is done by collecting data at two moments (t). The first moment is before an experimental event occurred and the second one is after this occurrence. Both groups are carefully selected.
Though the term group is used, nothing has been said about the size of the group; the minimum could be even 1 so it is even applicable on qualitative research or on comparing two case studies.
Using a placebo
It is not ethical to withhold people medicine when they are ill. But if you give them the same treatment too, the effect of the treatment cannot be tested. Then the control group should get the treatment as usual. In the design this can be stated as:
t1 t2
Group A R or M O1 X O2
Group B R or M O1 TaU O2
The effect of the new medicine now is tested against the usual treatment.
Sometimes the medicine is expected to work, just because people know they are selected for a special treatment. Now the effect of the treatment is mixed up with the expectations of the members. To unravel this clutching, a placebo is used. A placebo is giving the member of a group a similar treatment but not the one of which an effect is expected. In the design this can be stated as:
t1 t2
Group A R or M O1 X O2
Group B R or M O1 P O2
Related topics to clinical research design:
- Design
- Explorative research
- Descriptive research
- Comparative research
- Evaluation research
- Longitudinal research
- Random
- Matching
This manual helps you to do scientific research:
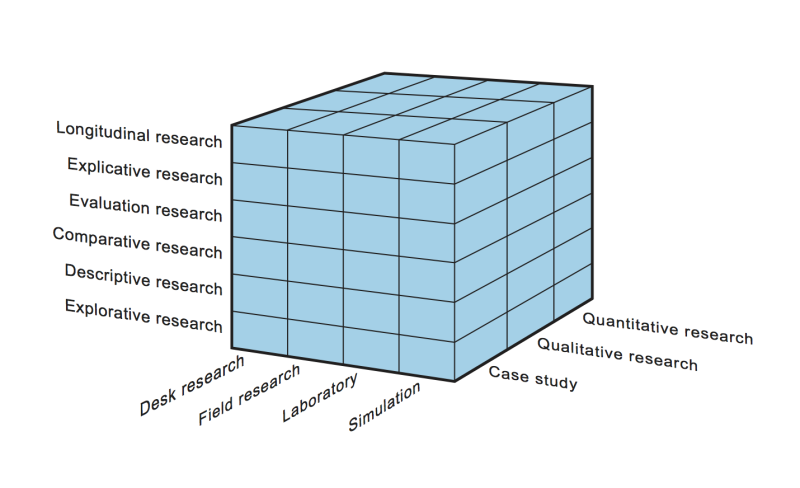
Every research can be classified on three dimensions:
1) the research design
2) the location of data collection and
3) the number of cases.
Together they form the research cube. With this classification 72 types of research can be distinguished.